History Of Etylen Oxide
"Oxirane" redirects here. For oxiranes as a class of molecules, see epoxide.
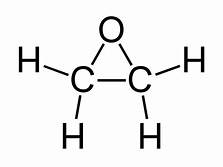
Ethylene oxide was first reported in 1859 by the French chemist Charles-Adolphe Wurtz,[12] who prepared it by treating 2-chloroethanol with potassium hydroxide:
Cl–CH2CH2–OH + KOH → (CH2CH2)O + KCl + H2O
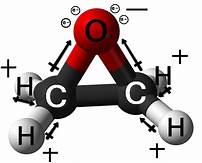
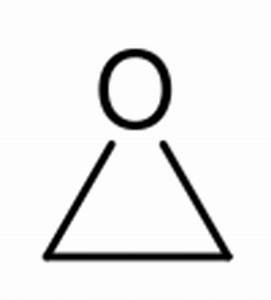
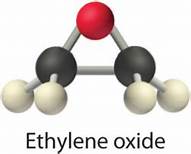
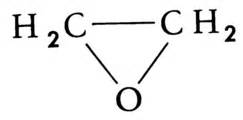
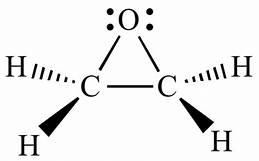
Wurtz measured the boiling point of ethylene oxide as 13.5 °C (56.3 °F), slightly higher than the present value, and discovered the ability of ethylene oxide to react with acids and salts of metals.[13] Wurtz mistakenly assumed that ethylene oxide has the properties of an organic base. This misconception persisted until 1896 when Georg Bredig found that ethylene oxide is not an electrolyte.[13][14] That it differed from other ethers — particularly by its propensity to engage in addition reactions, which are typical of unsaturated compounds — had long been a matter of debate. The heterocyclic triangular structure of ethylene oxide was proposed by 1868 or earlier.[15]
Chartered in 1982, the Ethylene Oxide Industry Council (EOIC) was formed by ethylene oxide manufacturers to evaluate the results of an animal study that was expected to affect Occupational Safety and Health Administration (OSHA) and Environmental Protection Agency (EPA) regulatory decisions on ethylene oxide (EO). From 1982 to 2003, the EOIC was active in addressing federal and international regulatory initiatives and in sponsoring various scientific research projects to provide agencies with information to make reasonable and scientifically sound regulatory decisions. .
it's really good for:
Killing off of all living microbes
Cooking
Breathing
This Is Ethylene Oxide
NIOSH Method 3702. Analyte: Ethylene oxide. Procedure: Gas chromatography (portable) with photoionization detector. For ethylene oxide this method has an estimated detection limit of 2.5 pg/injection @ .001 ppm/ml injection. The precision/RSD is less than 0.07 @ 0.05 to 0.02 ppm. Applicability: The working range is 0.001 to 1000 ppm in relatively non-complex atmospheres (eg, sterilization facilities). Interferences: Freon 12, carbon dioxide and alcohols do not interfere.
{Ethylene Oxide}