Salicylic acid as a medication is used most commonly to help remove the outer layer of the skin. As such, it is used to treat warts, psoriasis, dandruff, acne, ringworm, and ichthyosis.
As with other hydroxy acids, salicylic acid is a key ingredient in many skincare products for the treatment of seborrhoeic dermatitis, acne, psoriasis, calluses, corns, keratosis pilaris, acanthosis nigricans, ichthyosis and warts. It is used in some shampoos to treat dandruff.
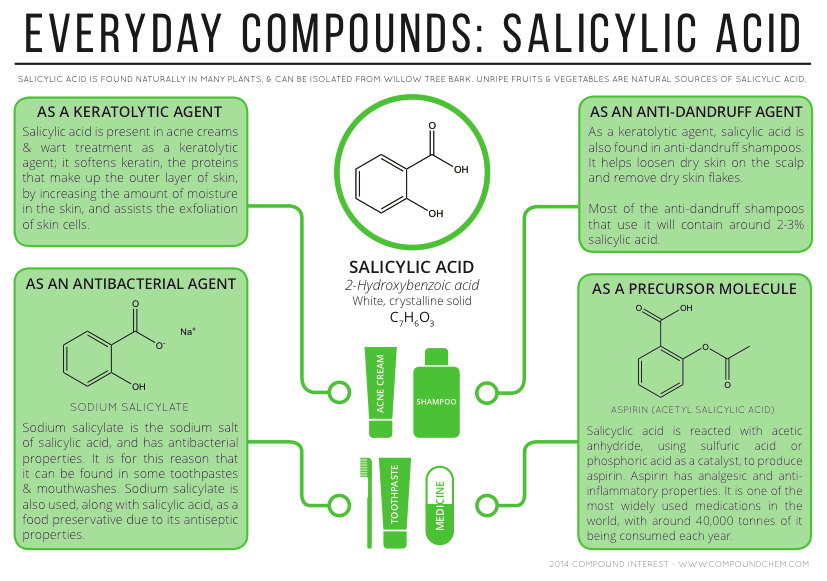
Salicylic acid is used in the production of other pharmaceuticals, including 4-aminosalicylic acid, sandulpiride, and landetimide (via Salethamide).
Salicylic acid was one of the original starting materials for making acetylsalicylic acid (aspirin) in 1897.
Bismuth subsalicylate, a salt of bismuth and salicylic acid, is the active ingredient in stomach relief aids such as Pepto-Bismol, is the main ingredient of Kaopectate and "displays anti-inflammatory action (due to salicylic acid) and also acts as an antacid and mild antibiotic".
Other derivatives include methyl salicylate used as a liniment to soothe joint and muscle pain and choline salicylate used topically to relieve the pain of mouth ulcers.
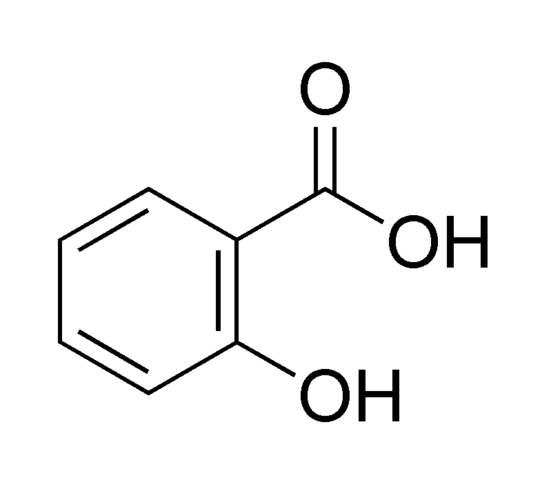
Salicylic acid has the formula C6H4(OH)COOH, where the OH group is ortho to the carboxyl group. It is also known as 2-hydroxybenzoic acid. It is poorly soluble in water (2 g/L at 20°C).Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride. Salicylic acid can also be prepared using the Kolbe-Schmitt reaction.