Ethanol
Reactions.
Ethanol is classified as a primary alcohol, meaning that the carbon its hydroxyl group attaches to has at least two hydrogen atoms attached to it as well. Many ethanol reactions occur at its hydroxyl group.
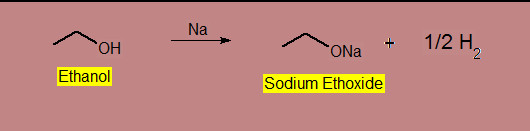
Reaction With Active Metals
The product will forms Alkoxied.
Using the reagent metals ; Na, Mg, Al, etc.
The reactivity of R-OH is 1o > 2o > 3o

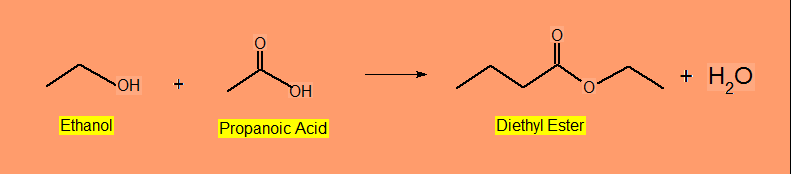
Esterification
Esterification ( Ester Formation ) involves cleavage of C-OH bond of the acid and of the O-H bond of the alcohol.
Example :
CH3CH2OH + CH3CH2OOH --> CH3CH2COOCH2CH3 + H2

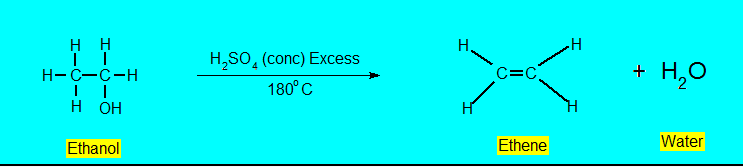
Dehydration
Dehydration reaction is using a sulphuric acid as the reagent.
- Alkane Formation
At 180oC alcohols undergo dehydration with concentrate H2SO4 to give alkanes.
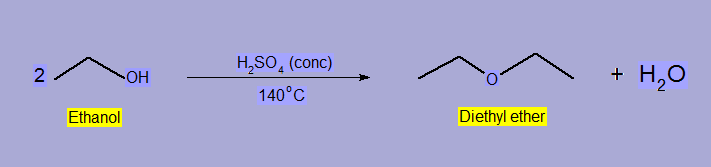
- Ether Formation
At 140oC alcohols undergo dehydration with concentrate H2SO4 to form ether.
- Alkyl Hydrogen Sulphate
At room temperature alcohols react with concentrate H2SO4 to form alkyl hydrogen sulphate.
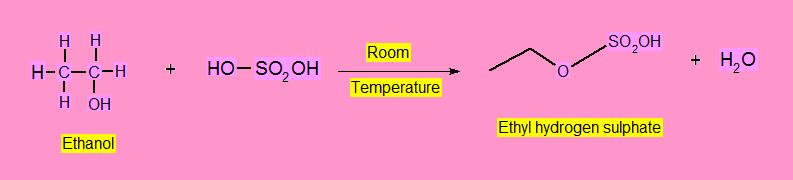



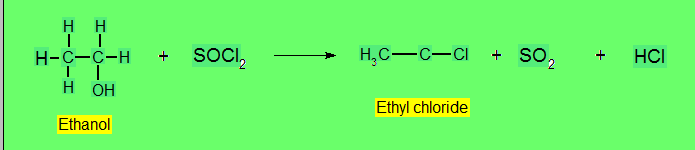
Alkylhalides Formation
- Reaction with Thionyl Chloride (SOCl3)
- Reaction with Phosphorus Halides (PX3 or PX5)
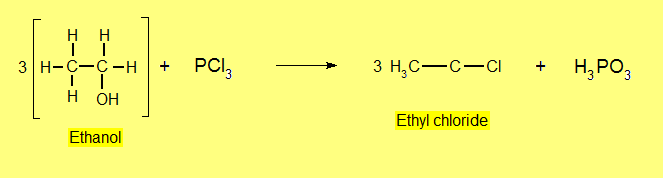
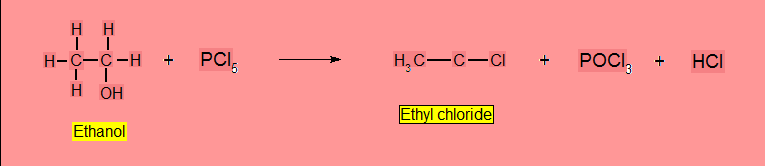
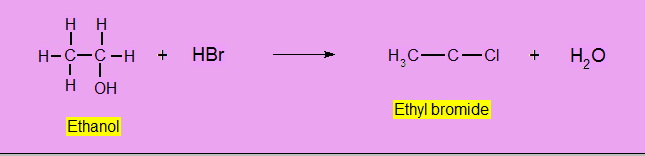
Reaction with Hydrogen Halides (HX)
HCl reacts with alcohols only in the presence of catalyst (anhydrous ZnCl2). No catalyst is required in the case of HBr or HI.
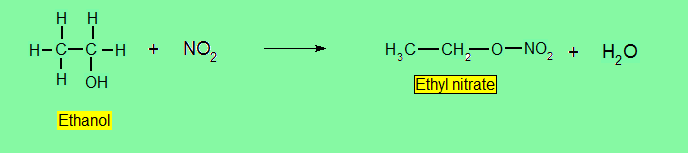
Reaction with nitric acid (HNO3)
The product from this reactions are formed Alkyl nitrates.
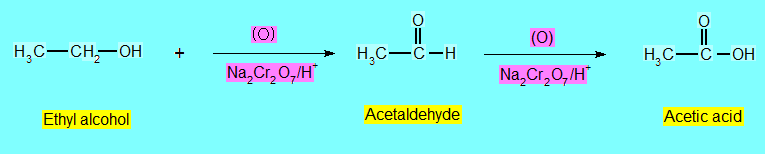
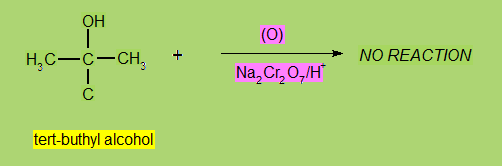
Oxidation
Differents types of alcohols give different products on oxidation.
Most widely used oxidizing agents are
KMnO4 + H2SO4 and Na2Cr2O7 + H2SO4.
- Primary Alcohols are oxidized to aldehydes.
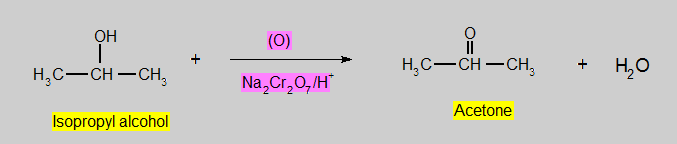
- Secondary Alcohols are oxidized to ketones.
- Tertiary Alcohols are don't undergo oxidation under normal conditions.
Contact.
Do you want to know about ethanol? Fill out the form and fill me in with the details :) We love meeting new people!



HCl reacts with alcohols only in the presence of catalyst (anhydrous ZnCl2). No catalyst is required in the case of HBr or HI.


- Primary Alcohols are oxidized to aldehydes.
- Secondary Alcohols are oxidized to ketones.
- Tertiary Alcohols are don't undergo oxidation under normal conditions.



Contact.
Do you want to know about ethanol? Fill out the form and fill me in with the details :) We love meeting new people!
