Ethanol
Chemistry.
Chemical Properties
Chemical Formula
Ethanol is a 2-carbon alcohol. Its molecular formula is CH3CH2OH. An alternative notation is CH3−CH2−OH, which indicates that the carbon of a methyl group (CH3−) is attached to the carbon of a methylene group (−CH2–), which is attached to the oxygen of a hydroxyl group (−OH). It is a constitutional isomer of dimethyl ether. Ethanol is sometimes abbreviated as EtOH, using the common organic chemistry notation of representing the ethyl group (C2H5−) with Et.
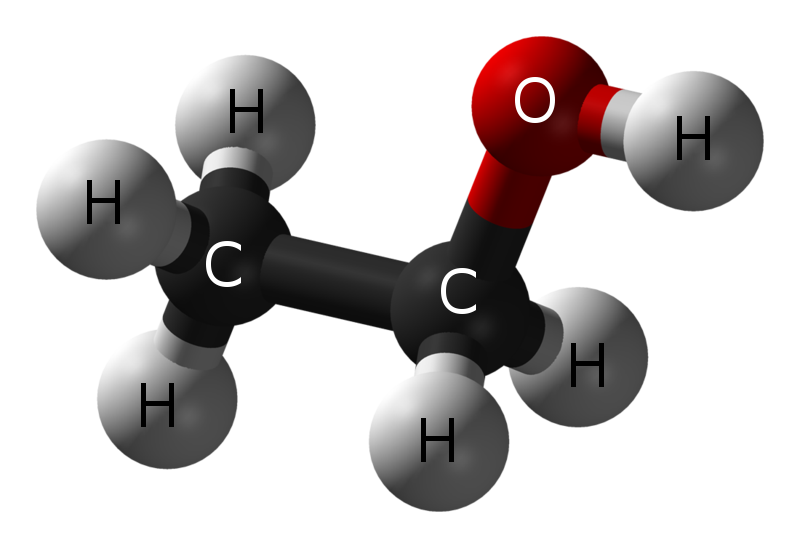
Ethanol, like all alcohols, has an OH (hydroxylic) group attached to a saturated carbon atom.
Calories
1 gram of ethanol provides 7 Calories (kilocalories) of the “metabolizable energy” or 6.1 Calories of the “net metabolizable energy.
Physical Properties
Appearance, Odor and Taste
At room temperature, ethanol is a clear, colorless, volatile liquid with a characteristic odor. When diluted, it is somewhat sweet, but concentrated alcohol has a strong, burning taste.
Solubility
Ethanol is highly soluble in water and organic solvents, but poorly soluble in fats and oils.
Density
Density of ethanol at 68 °F (20 °C) is 0.789 g/mL.
pH
Pure ethanol is neutral (pH ~7).Most alcoholic beverages are more or less acidic: table wine pH = 3.3-3.7 ,beer pH ~ 4.
Boiling Point
Boiling point of ethanol is 173.3 °F (78.5 °C)
Melting Point
Melting point of ethanol is -173.4 °F (-114.1 °C).
Contact.
Do you want to know about ethanol? Fill out the form and fill me in with the details :) We love meeting new people!
