Ethanol
Production.
Ethanol is produced both as a petrochemical, through the hydration of ethylene and, via biological processes, by fermenting sugars with yeast. Which process is more economical depends on prevailing prices of petroleum and grain feed stocks. In the 1970s most industrial ethanol in the United States was made as a petrochemical, but in the 1980s the United States introduced subsidies for corn based ethanol and today it is almost all made from that source.
Ethylene hydration
Ethanol for use as an industrial feedstock or solvent (sometimes referred to as synthetic ethanol) is made from petrochemical feed stocks, primarily by the acid-catalyzed hydration of ethylene:
C2H4 + H2O → CH3CH2OH
The catalyst is most commonly phosphoric acid, adsorbed onto a porous support such as silica gel or diatomaceous earth. This catalyst was first used for large-scale ethanol production by the Shell Oil Company in 1947. The reaction is carried out in the presence of high pressure steam at 300 °C (572 °F) where a 5:3 ethylene to steam ratio is maintained. In the U.S., this process was used on an industrial scale by Union Carbide Corporation and others, but now only LyondellBasell uses it commercially.
In an older process, first practiced on the industrial scale in 1930 by Union Carbide, but now almost entirely obsolete, ethylene was hydrated indirectly by reacting it with concentrated sulfuric acid to produce ethyl sulfate, which was hydrolyzed to yield ethanol and regenerate the sulfuric acid:
C2H4 + H2SO4 → CH3CH2SO4HCH
3CH2SO4H + H2O → CH3CH2OH + H2SO4
From CO2
CO2 can also be used as the raw material.
CO2can be converted using such organisms as Clostridium ljungdahlii, Clostridium autoethanogenum or Moorella sp. HUC22-1.
CO2can be converted using electrochemical reactions at room temperature and pressure.
From lipids
Lipids can also be used to make ethanol and can be found in such raw materials such as algae.
Fermentation
Main article: Ethanol fermentation
See also: Yeast in winemaking
Ethanol in alcoholic beverages and fuel is produced by fermentation. Certain species of yeast (e.g., Saccharomyces cerevisiae) metabolize sugar, producing ethanol and carbon dioxide. The chemical equations below summarize the conversion:
C6H12O6 → 2 CH3CH2OH + 2 CO2
C12H22O
11 + H2O → 4 CH3CH2OH + 4 CO2
Fermentation is the process of culturing yeast under favorable thermal conditions to produce alcohol. This process is carried out at around 35–40 °C (95–104 °F). Toxicity of ethanol to yeast limits the ethanol concentration obtainable by brewing; higher concentrations, therefore, are obtained by fortification or distillation. The most ethanol-tolerant yeast strains can survive up to approximately 18% ethanol by volume.
To produce ethanol from starchy materials such as cereal grains, the starch must first be converted into sugars. In brewing beer, this has traditionally been accomplished by allowing the grain to germinate, or malt, which produces the enzyme amylase. When the malted grain is mashed, the amylase converts the remaining starches into sugars.
Cellulose
Sugars for ethanol fermentation can be obtained from cellulose. Deployment of this technology could turn a number of cellulose-containing agricultural by-products, such as corncobs, straw, and sawdust, into renewable energy resources. Other agricultural residues such as sugar cane bagasse and energy crops such as switchgrass may also be a sources of fermentable sugars.
Testing
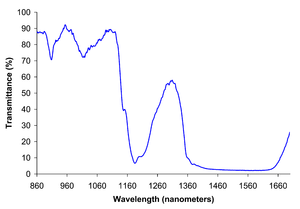
EInfrared reflection spectra of liquid ethanol, showing the -OH band centered at ≈3300 cm−1 and C-H bands at ≈2950 cm−1..
Near infrared spectrum of liquid ethanol.
Breweries and biofuel plants employ two methods for measuring ethanol concentration. Infrared ethanol sensors measure the vibrational frequency of dissolved ethanol using the CH band at 2900 cm−1. This method uses a relatively inexpensive solid state sensor that compares the CH band with a reference band to calculate the ethanol content. The calculation makes use of the Beer-Lambert law. Alternatively, by measuring the density of the starting material and the density of the product, using a hydrometer, the change in specific gravity during fermentation indicates the alcohol content. This inexpensive and indirect method has a long history in the beer brewing industry.
Contact.
Do you want to know about ethanol? Fill out the form and fill me in with the details :) We love meeting new people!

