 Chemical.ly ™
Chemical.ly ™
Phenol can not be oxidized into aldehydes or ketones whose number of C atoms is the same, because the OH group is attached to an atom C which does not bind to the H atom anymore. Thus phenol can be likened to tertiary alkanol
 Chemical.ly ™
Chemical.ly ™
Phenol can not be oxidized into aldehydes or ketones whose number of C atoms is the same, because the OH group is attached to an atom C which does not bind to the H atom anymore. Thus phenol can be likened to tertiary alkanol
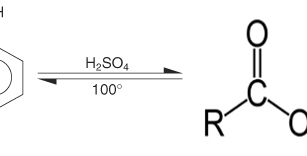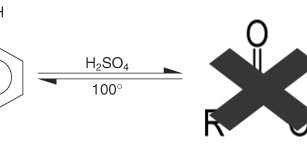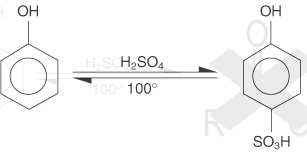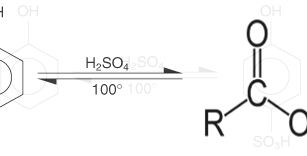
If reacted with concentrated H2SO4 does not form ester but form phenolsulfonic acid (o or p)
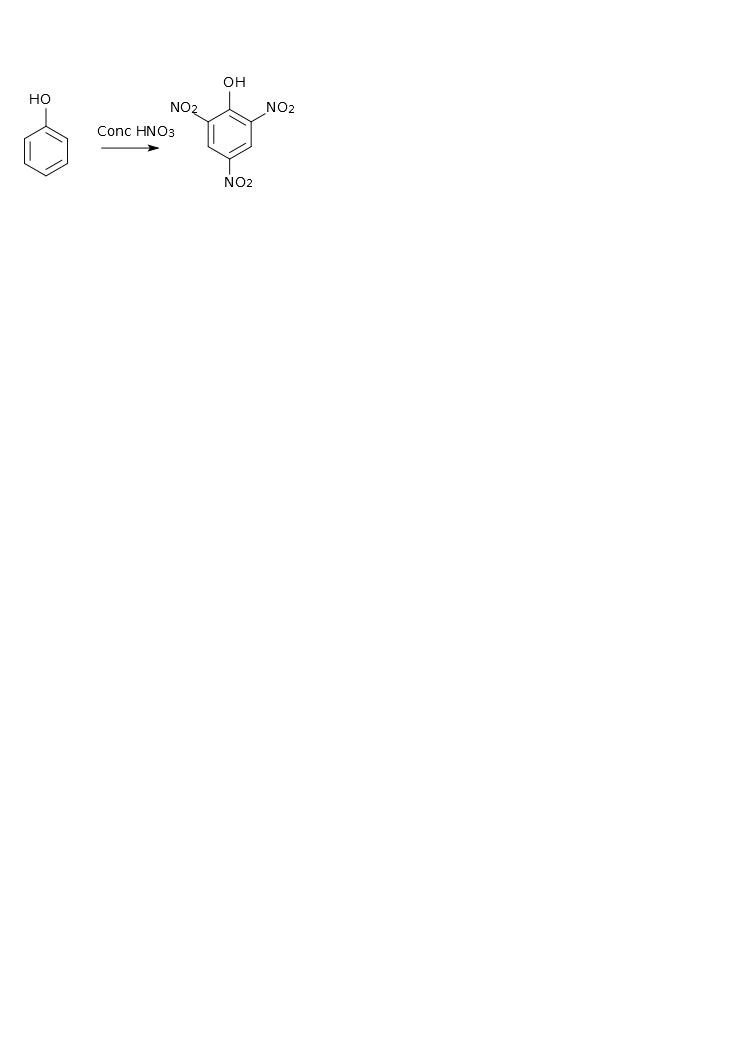
With concentrated HNO3 produced nitrophenol and at subsequent nitration formed 2,4,6 trinitrofenol or picrat acid
The phenol solution in water is as weak acid so ionize as follows: Therefore phenol can react with the base and form phenolic salts