
Synthetic vanillin became more readily available in the 1930s, when the production of clove oil was replaced by the production of lignin-containing wastes produced by sulphite pulping processes to make pulp for the paper industry. In 1981, single made pulp and paper mills that supply 60% of the world market for synthetic vanillin in Ontario. However, subsequent developments in the pulp industry have made the lignin waste less attractive in the industry as a raw material for vanillin sitensis. While some vanillins are still made from lignin waste, most synthetic vanillin is now synthesized in the two-stage prodses of pentochemical precursors, guaiacol and glyoxylic acid.
Beginning in 2000, Rhodia began to market biosynthetic vanillin made by the action of microorganisms on ferulic acid extracted from rice grains. At a price of Rp 5,974,500 / kg (at an exchange rate of Rp 8,535 / dollar), this product was sold under the trademark name Rhovanil Natural, is not a competitive cost with petrochemical vanillin, which sells for about $ 15 / kg (Rp 128.000 / kg) However, unless vanillin is synthesized from lignin or guaiacol, it can be labeled as a natural flavor enhancer.
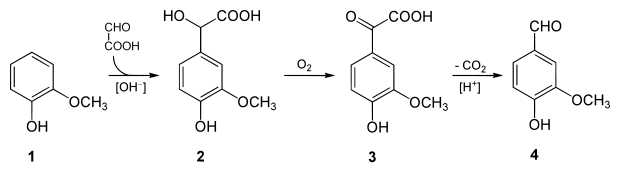
The demand for vanilla flavoring has long exceeded the supply of vanilla beans. As of 2001, the annual demand for vanillin was 12,000 tons, but only 1,800 tons of natural vanillin were produced.The remainder was produced by chemical synthesis. Vanillin was first synthesized from eugenol (found in oil of clove) in 1874–75, less than 20 years after it was first identified and isolated. Vanillin was commercially produced from eugenol until the 1920s. Later it was synthesized from lignin-containing "brown liquor", a byproduct of the sulfite process for making wood pulp. Counterintuitively, though it uses waste materials, the lignin process is no longer popular because of environmental concerns, and today most vanillin is produced from the petrochemical raw material guaiacol. Several routes exist for synthesizing vanillin from guaiacol. At present, the most significant of these is the two-step process practiced by Rhodia since the 1970s, in which guaiacol (1) reacts with glyoxylic acid by electrophilic aromatic substitution.The resulting vanillylmandelic acid (2) is then converted via 4-Hydroxy-3-methoxyphenylglyoxylic acid (3) to vanillin (4) by oxidative decarboxylation.
this is an example of synthesis vanillin from some product
Synthesis vanillin from vanilla sugar
