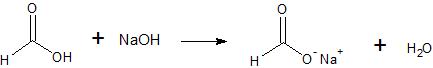
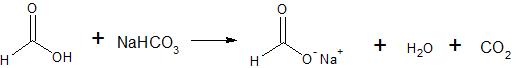
The relative acidity of Formic Acid mean that Formic Acid react readily with aqueous solutions of sodium hydroxide and sodium bicarbonate to form soluble sodium salts
Water-insoluble Formic Acid dissolve in either aqueous sodium hydroxide or aqueous sodium bicarbonate.
Lithium aluminum hydride (LiAlH4, abbreviated LAH) reduces Formic Acid to Methanol.
Formic Acid is more difficult to reduce than aldehydes and ketones. LAH, however, is a strong enough reducing agent to accomplish this transformation. Sodium borohydride (NaBH4), which we shall discuss shortly, is commonly used to reduce aldehydes and ketones, but it is not strong enough to reduce carboxylic acids (Formic Acid).
Great care must be taken when using LAH to avoid the presence of water or any other weakly acidic solvent (e.g., alcohols). LAH reacts violently with proton donors to release hydrogen gas. Anhydrous diethyl ether (Et2O) is a commonly used solvent for LAH reductions. After all of the LAH has been consumed by the reduction step of the reaction, however, water and acid are added to neutralize the resulting salts and facilitate isolation of the alcohol products.
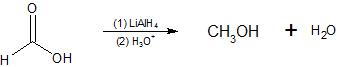
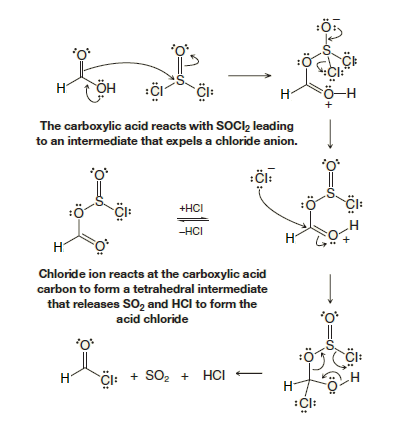
Since acyl chlorides are the most reactive of the acid derivatives, we must use special reagents to prepare them. We use other acid chlorides, the acid chlorides of inorganic acids: PCl5 (an acid chloride of phosphoric acid), PCl3 (an acid chloride of phosphorous acid), and SOCl2 (an acid chloride of sulfurous acid). All of these reagents react with Formic Acid to give acyl chloridesMethanoyl Chloride in good yield:
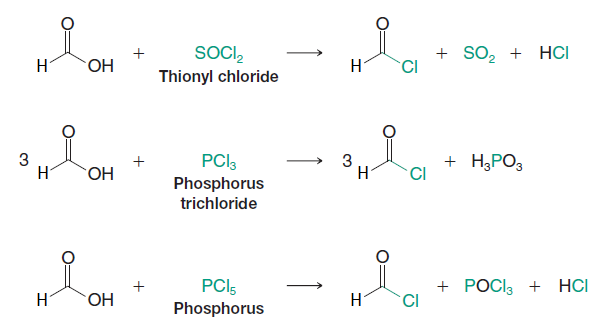
These reactions all involve nucleophilic addition–elimination by a chloride ion on a highly reactive intermediate: a protonated acyl chlorosulfite, a protonated acyl chlorophosphite, or a protonated acyl chlorophosphate. These intermediates contain even better acyl leaving groups than the acyl chloride product. Thionyl chloride, for example, reacts with a carboxylic acid in the following way:
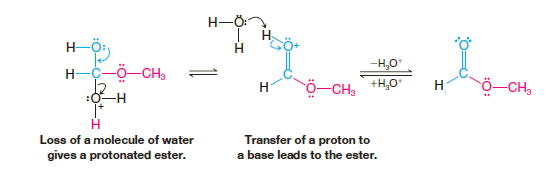
Formic acid reacts with alcohols to form esters through a condensation reaction known as esterification (Ester formation):
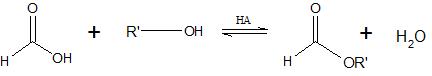
Fischer esterifications proceed very slowly in the absence of strong acids, but they reach equilibrium within a matter of a few hours when an acid and an alcohol are refluxed with a small amount of concentrated sulfuric acid or hydrogen chloride. Since the position of equilibrium controls the amount of the ester formed, the use of an excess of either the carboxylic acid or the alcohol increases the yield based on the limiting reagent. Just which component we choose to use in excess will depend on its availability and cost. The yield of an esterification reaction can also be increased by removing water from the reaction mixture as it is formed.
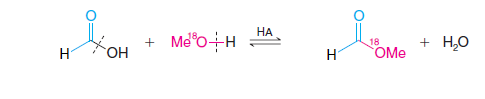
When Formic Acid reacts with methanol that has been labeled with 18O, the labeled oxygen appears in the ester. This result reveals just which bonds break in the esterification:
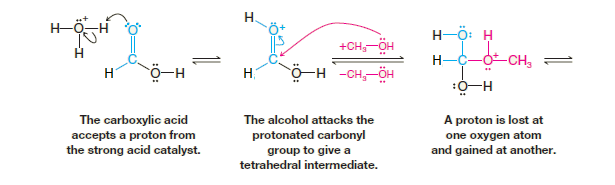
The results of the labeling experiment and the fact that esterifications are acid catalyzed are both consistent with the mechanism that follows. This mechanism is typical of acid-catalyzed nucleophilic addition–elimination reactions at acyl carbon atoms. If we follow the forward reactions in this mechanism, we have the mechanism for the acid-catalyzed esterification of an acid.
Formic Acid reacts with aqueous ammonia to form ammonium salts:
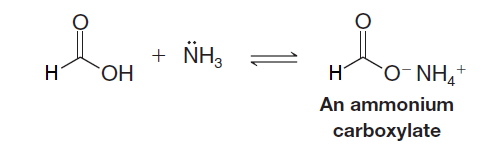
Because of the low reactivity of the methanoate ion toward nucleophilic addition–elimination, further reaction does not usually take place in aqueous solution. However, if we evaporate the water and subsequently heat the dry salt, dehydration produces an amide:
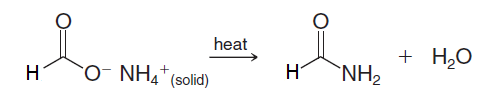
This is generally a poor method for preparing amides. A much better method is to convert the acid to an acyl chloride and then treat the acyl chloride with ammonia or anamine. Amides are of great importance in biochemistry. The linkages that join individual amino acids together to form proteins are primarily amide linkages. As a consequence, much research has been done to find convenient and mild ways for amide synthesis. Dialkylcarbodiimides, such as diisopropylcarbodiimide and dicyclohexylcarbodiimide (DCC), are especially useful reagents for amide synthesis. Dialkylcarbodiimides promote amide formation by reacting with the carboxyl group of an acid and activating it toward nucleophilic addition–elimination.
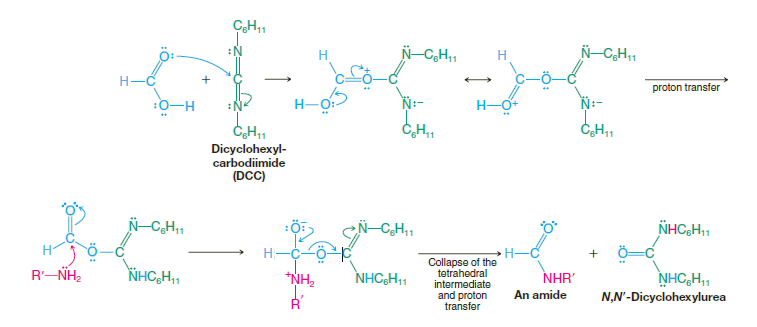
The intermediate in this synthesis does not need to be isolated, and both steps take place at room temperature. Amides are produced in very high yield.