Synthesis of 2-Hexanone
Preparation of 2-hexanone
1. Oxidation of Secondary Alcohols
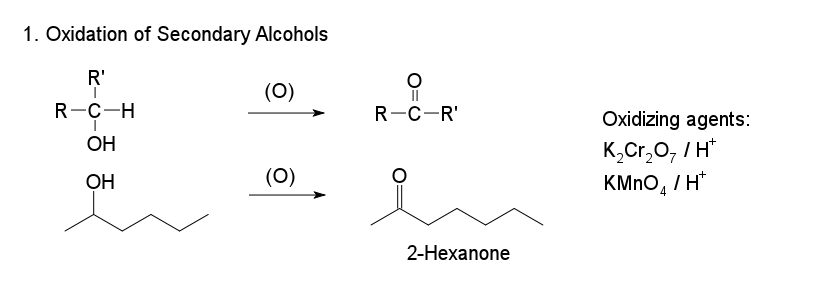
The oxidation of secondary alcohols to ketones may be carried out using strong oxidizing agents, because further oxidation of a ketone occurs with great difficulty. Normal oxidizing agents include potassium dichromate (K2Cr2O7) and chromic acid (H2CrO4). The conversion of 2‐propanol to 2‐propanone illustrates the oxidation of a secondary alcohol.
Where a secondary alcohol is oxidised, it is converted to a ketone. The hydrogen from the hydroxyl group is lost along with the hydrogen bonded to the second carbon. The remaining oxygen then forms double bonds with the carbon. This leaves a ketone, Ketones cannot normally be oxidised any further because this would involve breaking a C–C bond, which requires too much energy.
2. Catalytic Dehydrogenation of Secondary Alcohols
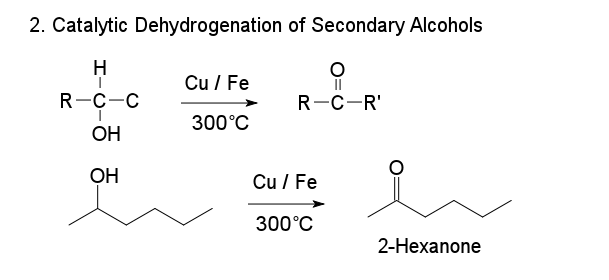
That preparation have the same way with Oxidation of secondary alcohols to make the ketone. The different between those preparation is Catalytic Dehydrogenation of Secondary Alcohols using metal ions.
3. Ozonolysis of Alkenes
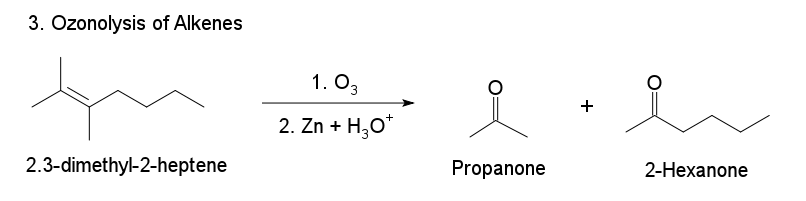
When one or both alkene carbons contain two alkyl groups, ozonolysis generates one or two ketones. The ozonolysis of 2,3‐dimethyl-2-heptene produces both propanone (a ketone) and 2-Hexanone (a ketone).
4. By Alkaline Hydrplysis of Gem-Dihalides
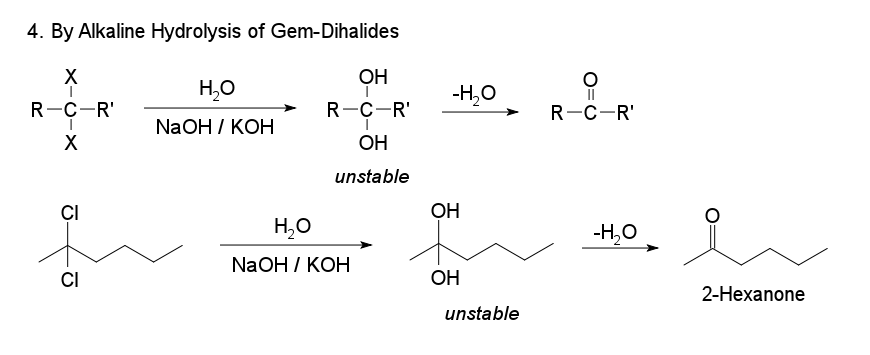
Ketones are obtained by the alkaline hydrolysis of gem dihalides in which the two halogen atoms are not attached to the terminal carbon atom.
5. Hydration of Alkynes
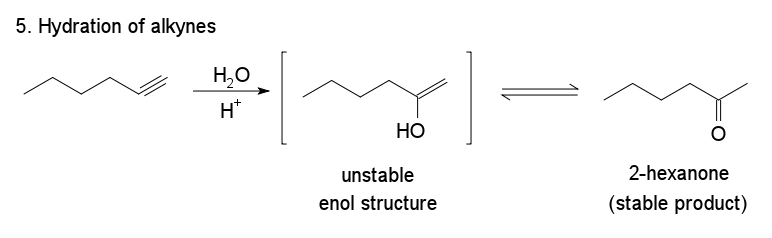
The addition of water to an alkyne leads to the formation of an unstable vinyl alcohol. These unstable materials undergo keto‐enol tautomerization to form ketones. The hydration of propyne forms 2‐propanone, as the following figure illustrates.
6. Grignard reagents
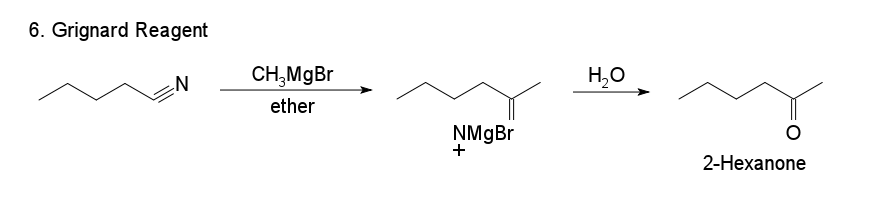
Hydrolysis of the salt formed by reacting a Grignard reagent with a nitrile produces good ketone yields. For example, you can prepare acetone by reacting the Grignard reagent methyl magnesium bromide (CH 3MgBr) with methyl nitrile
How to make 2-Hexanone?
A mixture of 1 g of mercury(II) sulfate, 1 g of concentrated sulfuric acid, and 150 g of 70% methanol (or 150 g of 70% acetone or 50 g of 60% acetic acid) is warmed to 60° in a 500-ml three-necked flask fitted with a stirrer, dropping funnel, and reflux condenser. 1-Hexyne (41.0 g, 0.5 mole) is dropped in, with stirring, within 1 hour. The mixture is stirred for a further 3 hours at the temperature stated, then cooled, and worked up. The methanol (or acetone) is distilled off and the 2-hexanone is salted out from the residue by solid sodium chloride. The ketone layer is separated, washed, neutralized, dried over calcium chloride, and distilled, giving 78.8% of 2-hexanone, b.p. 120°. When acetic acid has been used as solvent, it is neutralized with sodium carbonate solution before working up as above.