Citric Acid
Citric acid is a weak organic acid that has the chemical formula C6H8O7. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.More than a million tons of citric acid are manufactured every year.
It is used widely as an acidifier, as a flavoring and chelating agent.A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate ion is written as C6H5 O3−7 or C3H5O(COO)3−.
Chemical Characteristics
Citric acid was first isolated in 1784 by the chemist Carl Wilhelm Scheele, who crystallized it from lemon juice.It can exist either in an anhydrous (water-free) form or as a monohydrate. The anhydrous form crystallizes from hot water, while the monohydrate forms when citric acid is crystallized from cold water. The monohydrate can be converted to the anhydrous form at about 78 °C. Citric acid also dissolves in absolute (anhydrous) ethanol (76 parts of citric acid per 100 parts of ethanol) at 15 °C. It decomposes with loss of carbon dioxide above about 175 °C.
Citric acid is normally considered to be a tribasic acid, with pKa values, extrapolated to zero ionic strength, of 5.21, 4.28 and 2.92 at 25 °C. The pKa of the hydroxyl group has been found, by means of 13C NMR spectroscopy, to be 14.4. Citric acid are buffer solutions between about pH 2 and pH 8. In biological systems around pH 7, the two species present are the citrate ion and mono-hydrogen citrate ion. The SSC 20X hybridization buffer is an example in common use. Tables compiled for biochemical studies are available.
On the other hand, the pH of a 1 mM solution of citric acid will be about 3.2. The pH of fruit juices from citrus fruits like oranges and lemons depends on the citric acid concentration, being lower for higher acid concentration and conversely.
Acid salts of citric acid can be prepared by careful adjustment of the pH before crystallizing the compound. See, for example, sodium citrate.The citrate ion forms complexes with metallic cations. The stability constants for the formation of these complexes are quite large because of the chelate effect. Consequently, it forms complexes even with alkali metal cations. However, when a chelate complex is formed using all three carboxylate groups, the chelate rings have 7 and 8 members, which are generally less stable thermodynamically than smaller chelate rings. In consequence, the hydroxyl group can be deprotonated, forming part of a more stable 5-membered ring, as in ammonium ferric citrate, (NH4)5Fe(C6H4O7)2·2H2O.
Esters such as triethyl citrate can be made.
In materials science, the Citrate-gel method is a process similar to the sol-gel method, which is a method for producing solid materials from small molecules. During the synthetic process, metal salts or alkoxides are introduced into a citric acid solution. The formation of citric complexes is believed to balance the difference in individual behaviour of ions in solution, which results in a better distribution of ions and prevents the separation of components at later process stages. The polycondensation of ethylene glycol and citric acid starts above 100ºС, resulting in polymer citrate gel formation.
Citric Acid Can Made In To Buffer
About Citric Acid
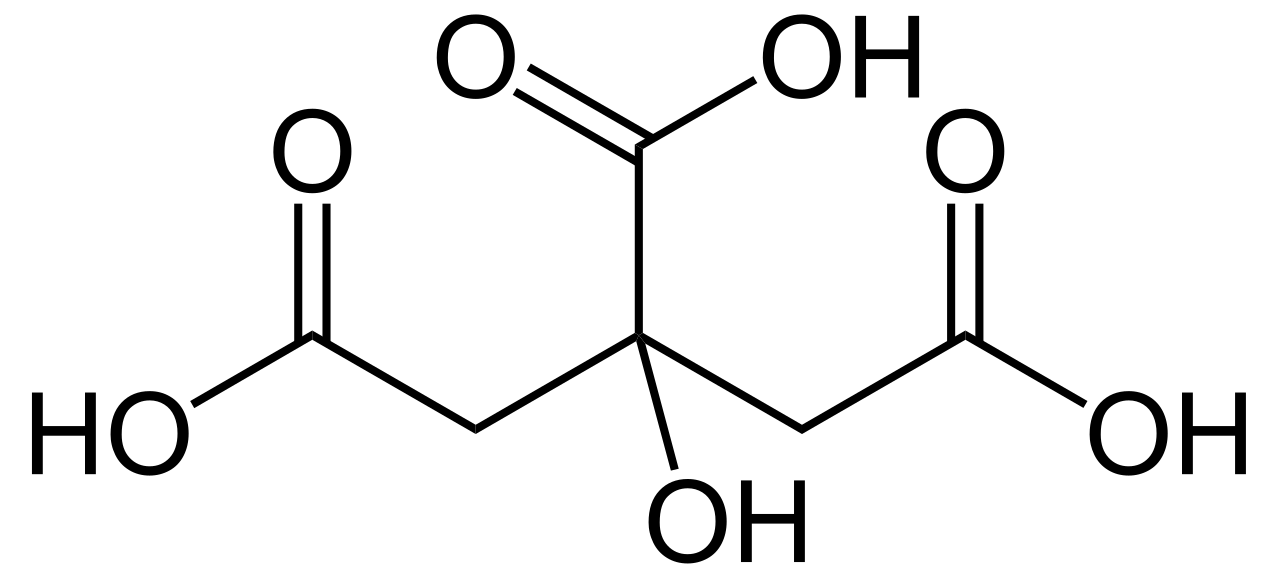
IUPAC Name: 2-Hydroxypropane-1,2,3-tricarboxylic acid
Chemical Formula: C6H8O7.
Appearance: Crystaline White Solid
Odor: Odorless
Density:1.665 g/cm3 (anhydrous)
Melting Point: 156 0 C
Boiling Point: 310 0 C
Solubility in Water: 547.79 g/100 mL (100 0 C)
Solubility: Soluble in Alcohol, Ether, Ethyl Acetate ;Insoluble in Hexene, Choroform, Carbon Sulfide, Toluene
Solubility in Ethanol: 62 g/100 g (25 0 C)
Solubility in Amylacetate: 4.41 g/100 g (25 0 C)
Solubility in Diethylether: 1.05 g/100 g (25 0 C)
Solubility in 1,4-Dioxane: 35.9 g/100 g (25 0 C)
log P : -1,64
Acidity (pKa1) : 3.13
Acidity (pKa2) : 4.76
Acidity (pKa3) : 6.40
Refractive index : 1.493–1.509 (20 0 C) or 1.46 (150 0 C)
Viscosity : 6.5 cP (50% aq. sol.)
Crystal Structure : Monoclinic
