STRUCTURE OF STEARIC ACID

To have a clear understanding of the structure of stearic acid, it is important to remember that carbon atoms can bind 4 atoms or other molecules. A carbon atom having 4 atoms or molecules attached to it is in its most stable form, making it relatively unreactive. The oxygen and hydrogen atoms are bonded to the tip carbon atom in such a way as to give charge, allowing it to form hydrogen bonds with other molecules. A hydrogen bond is a chemical bond formed between a hydrogen atom of one molecule and a negatively charged atom in another molecule. Some examples of atoms carrying negative charges are oxygen and nitrogen. The stearic acid molecule is bonded when the hydrogen bonds from the oxygen molecules are single bond bonded with hydrogen from the hydroxide group of the other molecule, so aldehyde groups formed. The parent chain of aldehyde is Stearaldehyde.
In drawing the structure of organic compounds, we can choose what type of structure we will use. there are several types of structures of organic compounds, as in the following example below.
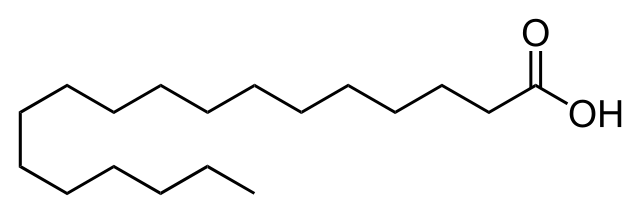
line structural diagram
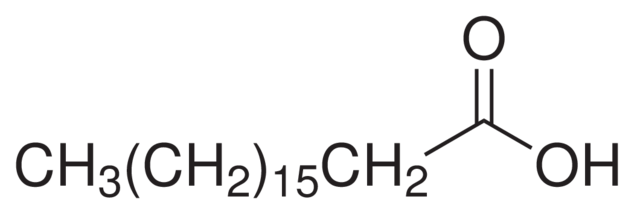
full condensed structural diagram
© 2018 Maintained by Samsul Maulana, All Right Reserved