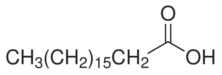Preparation Of Stearic Acid
Make Stearic Acid From Palm Oil
Stearic Acid can made from palm oil or CPO with two processes .
First is hydrolysis of palm oil without catalyst with pressure 45atm in temperature 250°C. Splitting conversion palm oil (triglyceride) can reach maximum 98%. In this hydrolysis Reaction of palm oil will produce myristic acid, palmitic acid, linoleic acid, oleic acid, stearic acid and glyserol. The percentage of stearic acid just about 5% from oil acid that formed, oleic acid 41% and linoleic acid 10%.
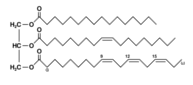
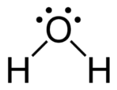
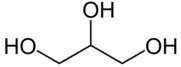
Hydrolysis of triglyceride with water :
 +
+  →
→  +
+ 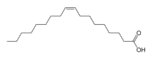
triglyceride + water → gliserol + oleic acid
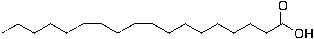
For getting economical stearic acid, oleic acid and linoleic acid must be hydrogenated. The second is hydrogenation reaction of oleic acid and linoleic acid and the stearic acid is formed with pressure 8atm in temperature 230°C and Ni is used as catalyst, conversion hydrogenation reaction is about 99%.
Hydrogenation of oleic acid and linoleic acid.
 +
+ 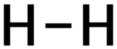 →
→ 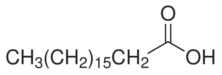
Oleic acid + H2 → Stearic Acid
 +
+ 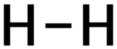 →
→ 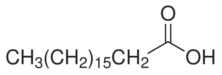
linoleic acid + H2 → Stearic acid
Diagram of The Process Stearic Acid is Made
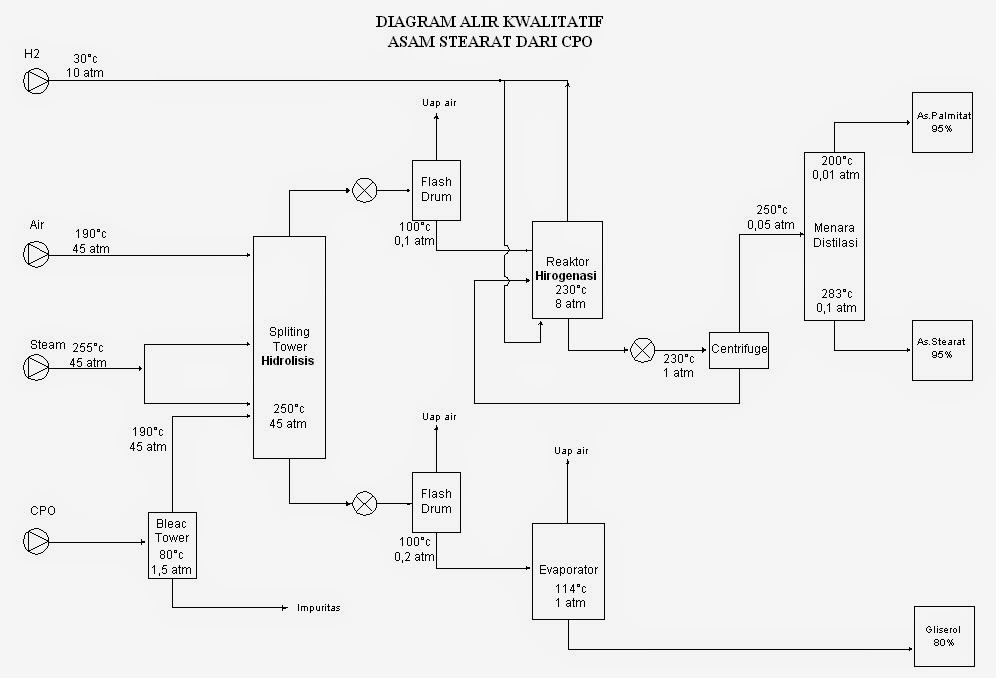
The Preparation of pure stearic acid is a complicated by the difficulty of removing final traces of the contaminating palmitic acid, since the separation by physical means is tedious and never and never unquestionably complete. In a recent study, Guy and Smith consided it necessary to subject "pure" stearic acid to twenty-four recrystallizations from various solvents and to three fractional distillations to obtain a 3.8% yield of final product, m. p. 69.62%, the homogently of which was still not beyond question. During the course of investigations of the chemistry of the fatty acids, we have found it convenient to prepare pure stearic acid totally free from palmitic acid by the catalytic reduction of readily purifiable octadecenoic acid (stearic acid), Elaidic acid, and especially the 𝝰- or β-eleostearic acid are easily available and may be brought to a high state of purity by a few recrystallizations of the free acids, while pure linoleic acid of theoretical iodine value may be obtained from crystalliziable 𝝰-tetrabromostearic acid. Quantitative reduction was effected by shaking the acetic acid solutions of the unsaturated acids for three hours in a an atmosphere of hydrogen at room temperature and 45 lb (3atm) pressure in the presence of platinum oxide catalyst.One recrystallization of the product from acetic acid or 85% alcohol yielded a stearic acid melting in a capillary tube at 69.9-70.2% and dissloving in concentrated sulfuric acid at 70°C without discoloration. A similiar procedure was used bt Francis, Collins and Piper for the preparation of behenic acid from erucic acid.
© 2018 Maintained by Davina Putri, All Right Reserved
+
 →
→  +
+ 
+
 →
→  +
+ 
 +
+ 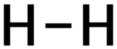 →
→ 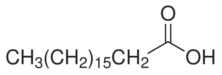
+
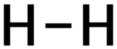 →
→