
Diethyl ether, or simply ether, is an organic compound in the ether class with formula (C2H5)2O,
Sometimes abbreviated as Et2O. It is a colorless, highly volatile flammable liquid,
It is commonly used as solven in laboratories and as a starting fluid for some engines.
It was formerly used as a general anestheic, until non-flammable drugs were developed, such as halothane.
It has been used as a recreational drug to cause intoxication.
Ether is a colorless, volatile liquid, distinctive odor irritate airway,
flammable / explosive, does not react with lime absorpent soda, and can decompose by air and light.
Ether is an anesthetic drug that is so strong that the patient can enter any level of anesthesia.
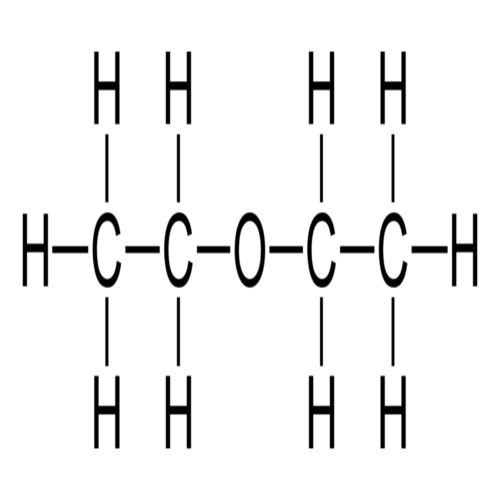
Ether is a chemical with an oxygen atom bonded to two alkyl Ether groups at room temperature and is usually colored with a sweet odor. The most common type of ether is diethyl ether, which is highly flammable and is one of the first anesthetics to be used in surgery Since the ether anesthetic effect is also used as a bland drug to produce sedation and euphoria Elet can also be used as a solvent to make perfumes. dissolve wax or other fats, or make other drugs. Ether is an important component of gasoline and additives in jet fuel and can be regarded as a source of fuel in the future.
The advantage of using ether is cheap and easy to obtain, it does not need to be used in conjunction with other drugs because it meets the anesthesia triad, is quite safe with wide security limits, and the tools used are quite simple. The disadvantages are easily explosive, unpleasant odor, airway irritation, salivary gland hypersecretion, cause nausea and vomiting, and may cause hyperglycemia. The amount of ether required depends on the body weight and condition of the patient, the need for anesthesia and the techniques used. Induction dose 10-20% by volume of vapor ether in oxygen or oxygen mixture and N2O. Dose of maintenance of stage III 5-15% vapor ether volume.