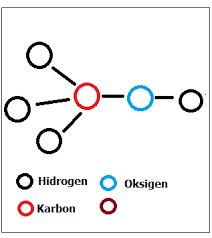



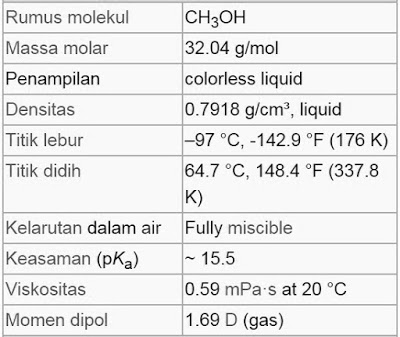
Metanol Chemical Formulas will share chemicals on the methanol chemical formula. As known that methanol or known as methyl alcohol, wood alcohol or spiritus, is a chemical compound with the chemical formula CH3OH. It is the simplest form of alcohol. In the atmosphere, it is a light, volatile, colorless, flammable, and toxic liquid with a distinctive odor (smells lighter than ethanol). methanol is used as an anti-frozen coolant, solvent, fuel and as an additive for industrial ethanol.
Methanol is produced naturally by anaerobic metabolism by bacteria. The result of the process is methanol vapor (in small amounts) in the air. After a few days, the methanol vapor will be oxidized by oxygen with the aid of sunlight to carbon dioxide and water. The chemical reactions of methanol that burn in the air and form carbon dioxide and water are as follows:
2 CH3 OH + 3 O2 ==> 2 CO2 + 4 H2 O
The fire from methanol is usually colorless. Therefore, we must be careful when near the burning methanol to prevent injury from unseen fires. Due to its toxic nature, methanol is often used as an additive for the manufacture of alcohol for industrial use; This "toxic" addition will keep the industry from wearable taxes because ethanol is the main ingredient for liquor (alcoholic beverages). Methanol is sometimes also called wood alcohol because it used to be a by-product of wood distillation. Methanol is currently produced by multi-stage proces natural gas and water vapor are burned in a furnace to form hydrogen and carbon monoxide gases; then, this hydrogen and carbon monoxide gas reacts in high pressure with the aid of a catalyst to produce methanol. The forming stage is endothermic and the synthesis stage is exothermic.